AARP Hearing Center

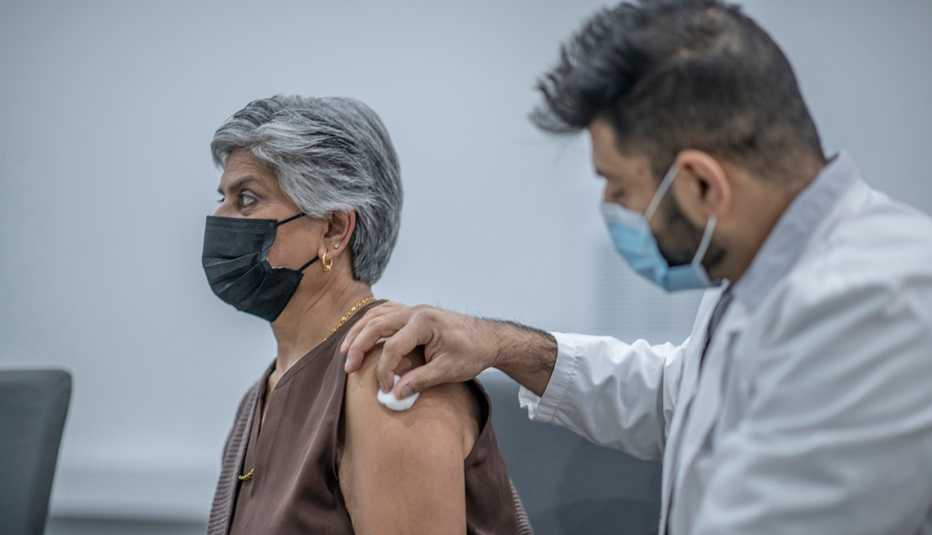
Americans now have access to a new batch of COVID-19 vaccines that are a closer match to many of the coronavirus variants currently circulating throughout the U.S.
Updated vaccines from Moderna and Pfizer-BioNTech received approval from the Food and Drug Administration (FDA) last month and are already available in many pharmacies and doctor’s offices throughout the country. And on Oct. 3, the FDA authorized a new version of the protein-based COVID-19 vaccine from Novavax. It will soon be available for people 12 and older.
Health officials are recommending that everyone 6 months and older get one of the three available options ahead of the fall and winter virus season.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, M.D., director of the FDA’s Center for Biologics Evaluation and Research, said in a news release. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness and manufacturing quality.”
The latest vaccines are designed to target the XBB.1.5 strain of omicron. While this particular variant is no longer driving the majority of the country’s infections, its close relatives are, and experts say the vaccines should provide good protection against them, including EG.5, or Eris. New data also suggests the shots will be effective against BA.2.86, a new variant that’s not yet widely circulating but recently grabbed the attention of scientists due to its multitude of mutations.
COVID-19 Shots at a Glace
What to know about the newly approved vaccines:
- All three of the updated vaccines are monovalent vaccines, meaning they target one strain of the virus, in this case XBB.1.5.
- It’s recommended that individuals 6 months and older get the latest COVID-19 shots at least two months after their last COVID vaccine. (Note: The Novavax vaccine is authorized for people 12 and older.)
- According to the FDA, side effects for the new COVID-19 vaccines are similar to previous versions of the mRNA vaccines.
The timing of the newly authorized vaccines coincides with an increase in COVID-19 hospitalizations and deaths, and experts suspect these trends will continue throughout the fall and winter when respiratory viruses usually prevail.
“We typically see a larger [COVID-19] spike occurring through January and February, and I don’t see any reason that won’t be the case here,” Cameron Wolfe, M.D., an infectious disease specialist at Duke Health and an associate professor at the Duke University School of Medicine, said in a recent media briefing. “So the timing of this release is actually really helpful. We know it takes a good few weeks to develop full antibody-related immunity. So getting patients the chance through October and into November of getting vaccinated, like we do with flu season, would be advantageous for the arrival of those waves in the winter.”
When will the new vaccines be available?
Many pharmacies and doctor’s offices are already stocked with the updated mRNA vaccines from Pfizer-BioNTech and Moderna. Novavax’s new vaccine is expected to be available in thousands of locations in the coming days, the company said on Oct. 3.
































































))
))




)
)





More From AARP
Is It Time to Start Wearing a Mask Again?
Experts share advice as COVID-19 cases start to climb3 Shots This Fall: What to Know About COVID, RSV and Flu Vaccines
Advice on when — and how often — to get themThese Are the 8 Vaccines You Need After 50
Are you up to date on all the recommended vaccines?