AARP Hearing Center

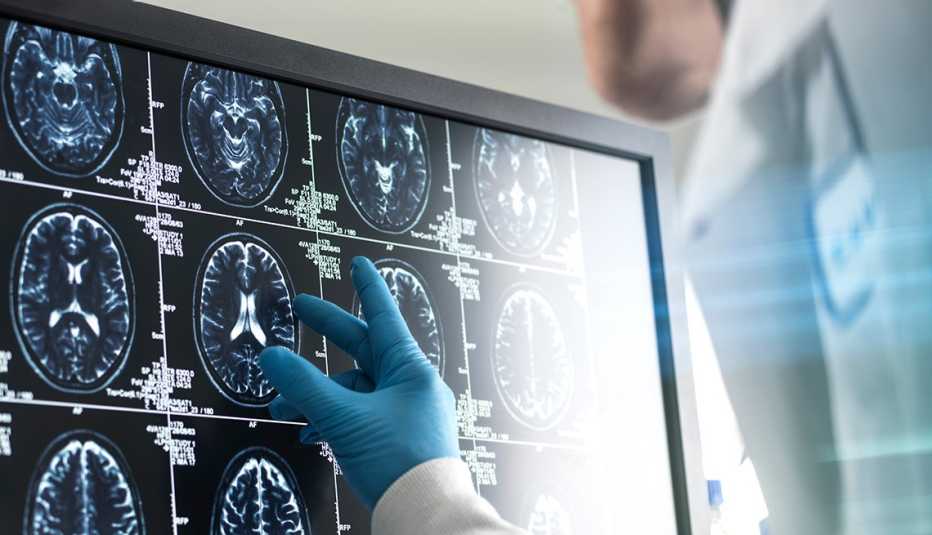
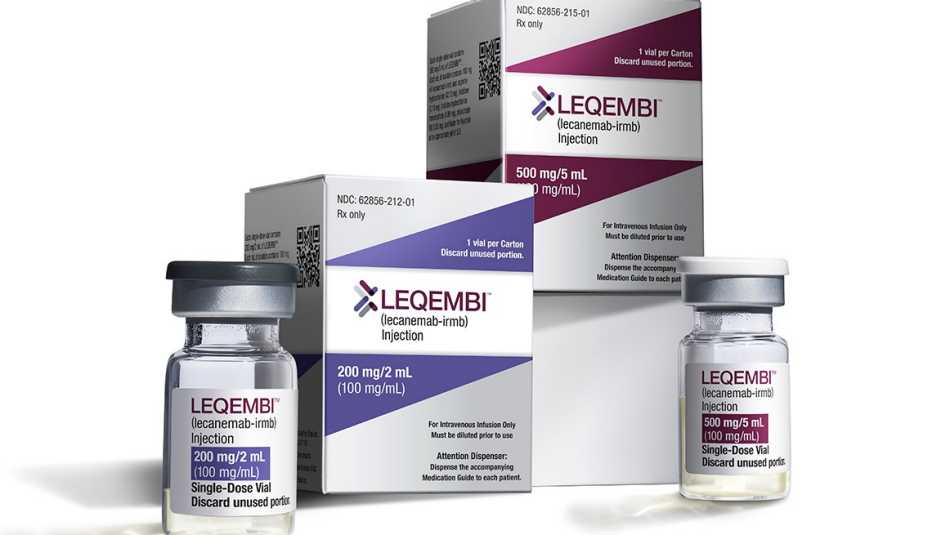
The U.S. Food and Drug Administration (FDA) has granted, for the first time, full, traditional approval for an Alzheimer’s medication that is designed to do more than treat the symptoms of the memory-robbing disease. The drug, lecanemab (brand name Leqembi), was shown in clinical trials to help slow the progression of Alzheimer’s, which affects more than 6.5 million older Americans and for which there is no cure.
“This is a big deal,” says Ronald Petersen, M.D., director of the Mayo Clinic Alzheimer’s Disease Research Center. Though there’s still work to do when it comes to finding medications that can treat Alzheimer’s, he says, “we’ve been waiting for this kind of breakthrough in the field for many, many years, and it’s a step in the right direction.”
The FDA granted lecanemab what’s known as accelerated approval in January. This fast-tracked approval process is reserved for drugs that treat serious conditions and appear promising based on preliminary evidence. In June, a committee of experts that advises the FDA voted in favor of fully approving lecanemab based on key findings from its large, late-stage clinical trial data.
The medication, a monoclonal antibody, significantly reduced the amount of amyloid in the brain. (Amyloid, a protein that clumps together to form plaques that disrupt cell function, is a hallmark of Alzheimer’s disease.) It also slowed the rate of cognitive decline — or the loss of thinking and memory skills — in people with early to mild Alzheimer’s disease by 27 percent over an 18-month period.
Essentially, that means the medication “may preserve the person’s level of function for a longer period of time,” Petersen says. “You’re not stopping the disease, so people do not actually stay at their precise level, but they progress much more slowly,” he adds, noting that this could be especially helpful for someone in the early stages of Alzheimer’s who is still able to work, pay bills and live independently.
“Today’s action is the first verification that a drug targeting the underlying disease process of Alzheimer’s disease has shown clinical benefit in this devastating disease,” Teresa Buracchio, acting director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release.
Another Alzheimer’s treatment that targets amyloid, called aducanumab (brand name Aduhelm), received accelerated approval from the FDA in 2021. However, this medication has not been fully approved.
Who can get the treatment?
Lecanemab is not for people with moderate or late-stage Alzheimer’s. The FDA has approved it only for people in the early stages (sometimes called mild cognitive impairment due to Alzheimer’s) and mild stages who have a confirmed presence of amyloid in the brain.



































































More From AARP
5 Simple Steps to Nourishing Your Brain
Use the S.H.A.R.P. method to kick-start a healthy way of eating
1 in 10 Older Americans Have Dementia
A new study highlights the burden in the U.S.
Make Brain-Boosting Habits Stick
These easy everyday behaviors can help boost your brain