AARP Hearing Center

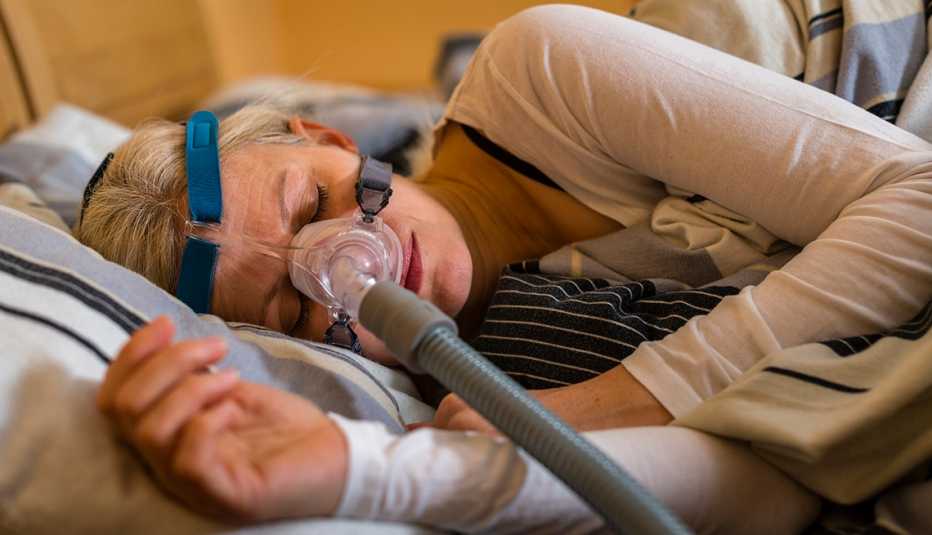
Consumers who used certain Philips Respironics CPAP, BiPAP or ventilator devices may qualify for a payout as part of a class action settlement stemming from claims that the foam used in the devices degraded and released harmful gases and particles into users’ airways, according to Angeion Group, a settlement administration company involved in the case.
Anyone who purchased, leased or rented one of the devices may receive compensation between $68 to $1,552 depending on the device and an additional $100 for each product returned to Philips.
Payment timing is uncertain due to ongoing legal proceedings, Angeion Group said in a document. A court hearing has been set for April 11 to decide whether to approve a potential settlement, and appeals may cause further payment delays.


What was behind the case?
In 2021, Philips recalled about 10.8 million sleep apnea devices; it has replaced approximately 2.5 million so far. Since then, the Food and Drug Administration (FDA) said it received over 105,000 complaints stemming from the products, including reports of at least 385 deaths.
Potential risks from exposure to the degraded foam include headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects, the FDA said in a previous warning to consumers.
How to file a claim
Those eligible for compensation should start the claims process here. The deadline to file a claim and send their device back to Philips Respironics is Aug. 9, 2024.
If you’re still using a recalled device, consult your physician before returning it to Philips.
The settlement doesn’t resolve claims for personal injury or medical monitoring and only applies to recalled devices that were purchased, leased or rented. It does not apply to remanufactured machines.
You may be able to receive a free remanufactured device or other benefits under Philips Respironics’ recall program by registering here.
Claims for the cost of personal injuries and medical expenses are not included in the settlement.
For free help with filing a claim, call the third-party settlement administrator at 855-912-3432 or send an email to info@RespironicsCPAP-ELSettlement.com. You may also use the serial number lookup tool or the eligibility determination page for additional help. For more information, visit the class actions frequently asked questions page.
The Philips devices cited in the case are:
- C-series S/T, AVAPS (C-series and C-series HT)
- DreamStation ASV
- DreamStation BiPAP
- DreamStation CPAP
- DreamStation Go
- DreamStation ST, AVAPS
- E30
- OmniLab Advanced Plus
- System One 50 Series ASV4 (Auto SV4)
- System One 50 Series Base
- System One 50 Series BiPAP
- System One 60 Series ASV4 (Auto SV4)
- System One 60 Series Base
- System One 60 Series BiPAP
- Trilogy 100/200, Garbin Plus, Aeris LiveVent
- V30 auto
Editor's note: An update on April 29, 2024, incorrectly associated a $1.1 billion Philips settlement with the class action suit mentioned in this article. However, the $1.1 billion settles a separate class action suit against Philips, addressing personal injury and medical monitoring losses linked to the recalled CPAP devices.





































































More From AARP
Product Recalls and Consumer Safety
The latest alerts impacting your health and home8 Major Health Risks for People 50 and Older
A look at the top killers — and how to dodge them
Know the Warning Signs of Sleep Apnea
This serious health condition has some surprising effects on everything from your blood pressure to your sex life