Staying Fit
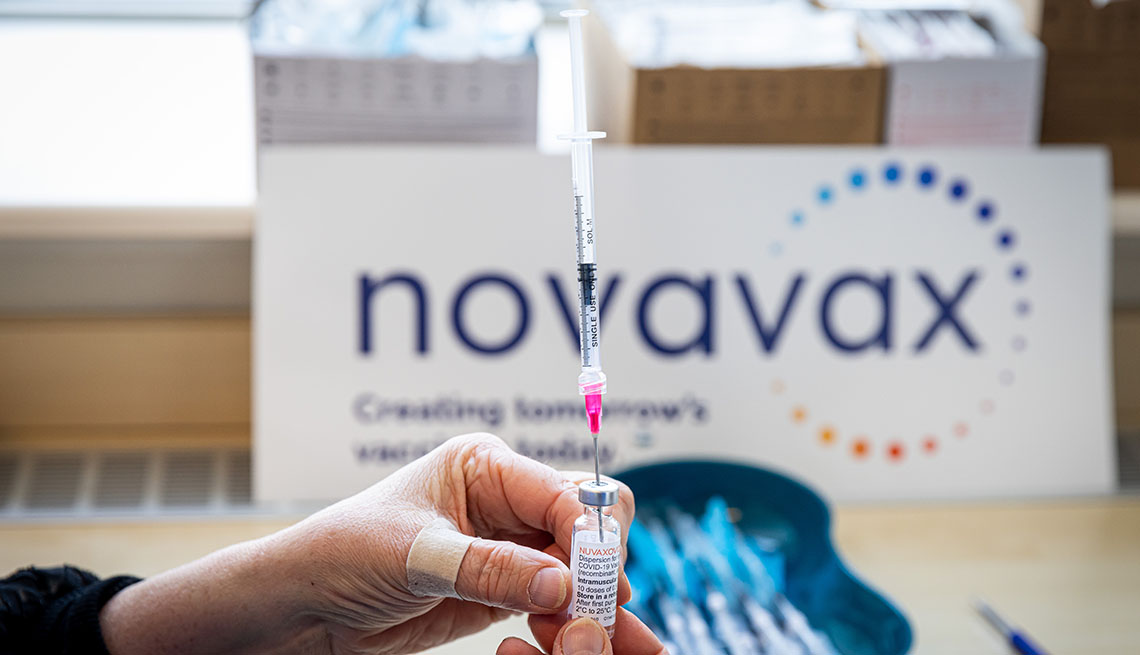
A new vaccine could soon be available to millions of U.S. adults who still have not been inoculated against COVID-19.
A panel of independent vaccine experts recommended on June 7 that the Food and Drug Administration (FDA) authorize a two-shot vaccine from the Maryland-based biotech Novavax. If the agency gives it the green light and the Centers for Disease Control and Prevention (CDC) signs off on its use, the vaccine would join three others that have been available to most U.S. adults for a little more than a year now.


AARP Membership— $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
But Novavax’s vaccine — which already has clearance in several other countries, including the U.K., Canada and Australia — is slightly different from the others being used in the U.S. Here’s what you need to know about the latest vaccine and how it might fit into the ongoing fight against COVID-19.
1. It uses a different technology
The main difference between the Novavax vaccine and the already-approved and widely used vaccines from Pfizer/BioNTech and Moderna is the technology behind the shots. Both Pfizer’s and Moderna’s vaccines use messenger RNA (mRNA) to deliver a blueprint of sorts to cells in the body. The cells then use this blueprint to make a harmless piece of the coronavirus’s signature spike protein, which the immune system flags as an invader and churns out antibodies to fight.
The third vaccine authorized for use in the U.S., made by Johnson & Johnson, is now only recommended in limited situations. It also instructs the body to build this protein to generate an immune response, only it delivers these instructions using a modified version of a different virus.
Novavax’s vaccine, on the other hand, takes a simpler, more traditional approach that’s been used for decades. Instead of prompting the body to make its own version of the spike protein, the protein is made in a lab and then delivered directly upon injection. Vaccines that help to fend off the flu, shingles and hepatitis B use this strategy, too.
Novavax’s vaccine also contains what’s called an adjuvant to give it extra oomph. Made from a type of tree bark, this ingredient helps boost antibody levels and create a stronger immune response. Matthew Frieman, an associate professor of microbiology and immunology at the University of Maryland School of Medicine, explains that the addition of an adjuvant is standard for plenty of vaccines. Shots given to prevent shingles and pneumonia as well as tetanus, diphtheria and pertussis (Tdap) all contain adjuvants.
Clinical trial data reviewed by the FDA’s advisory panel found that like the mRNA vaccines, Novavax’s shot was highly effective (about 90 percent) at preventing mild, moderate and severe COVID-19 against the original strain of the coronavirus.