AARP Hearing Center

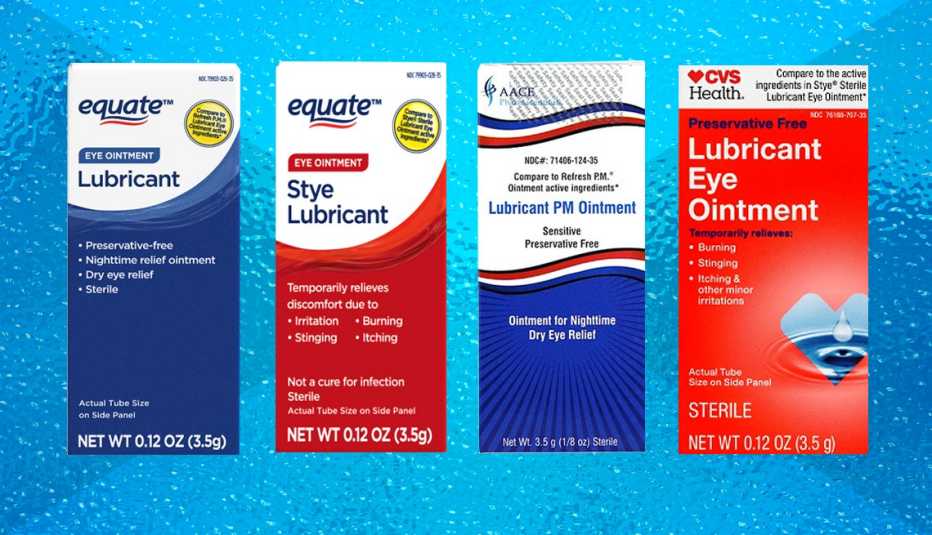
Four eye lubricant ointments, sold at Walmart and CVS stores and distributed to wholesalers nationwide, have been voluntarily recalled by the manufacturer over concerns the products may pose a “potential risk of eye infections or related harm,” according to a recall notice posted by the U.S. Food and Drug Administration.
FDA inspectors found a “lack of sterility assurance” at Brassica Pharma’s manufacturing facility in India. Eye medications are intended to be sterile, and contaminated products present an increased risk because they can bypass some of the body’s natural defenses, according to the FDA.
Consumers should stop using the products listed in the recall, which includes:
- Equate Lubricant Eye Ointment (sold at Walmart)
- Equate Style Lubricant Eye Ointment (sold at Walmart)
- CVS Health Lubricant Eye Ointment (sold at CVS)
- Lubricant PM Ointment (distributed by AACE Pharmaceuticals)
The products, which were also distributed nationwide to wholesalers, have expiration dates ranging from February 2024 to September 2025. See the full list of recalled products on the recall announcement page.
The company has not received any reports of the ointments having adverse effects on consumers.
Consumers should contact their physician or health care provider if they have experienced any problems that may be related to taking or using this product. Any adverse events or quality problems with any medication should be submitted to the FDA’s MedWatch Adverse Event Reporting program online at MedWatch or by faxing this form to 1-800-FDA-0178.



































































More From AARP
More Eye Drops Recalled Amid FDA Warning
Brands include, CVS, Rite Aid, Target
7 Mistakes You May Be Making With Eye Drops
How to keep your eyes healthy and free of infection
4 Types of Eye Drops and How to Use Them Safely
These can help with dryness, redness, allergies and irritation
Recommended for You