Staying Fit
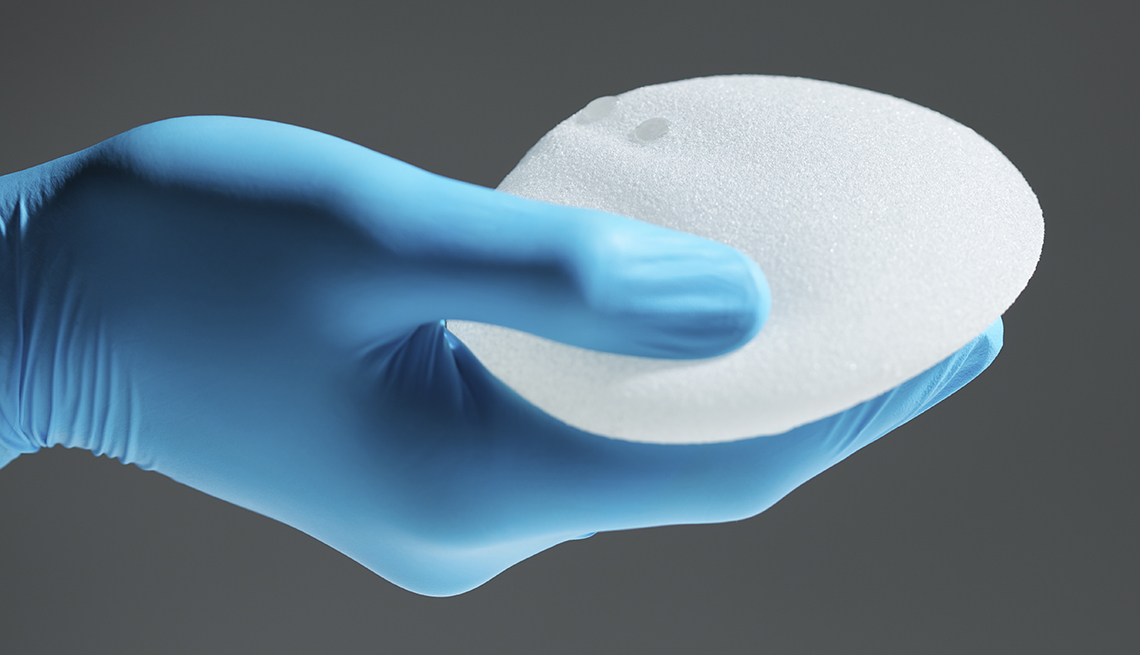
The U.S. Food and Drug Administration (FDA) is reporting that nine deaths have occurred as a result of a type of lymphoma associated with breast implants. The agency's just-released report also estimates that 457 related cancer diagnoses have been given between 2010 and September 2018.
While the disease appears to develop more frequently in individuals with textured implants than in those with smooth implants, the FDA said this week in a separate letter to health care providers that regardless of type — smooth or textured, or, in terms of filling, silicone or saline — all breast implants are associated with increased risk of anaplastic large cell lymphoma, or ALCL, a type of non-Hodgkin’s lymphoma.


AARP Membership— $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
The FDA first warned of the link between breast implants and ALCL in 2011. This new report is the latest in a series of findings that clarify that risk. A 2018 study in JAMA Oncology, for instance, found that women in the Netherlands with breast implants were also at a higher risk of developing ALCL, though their overall risk of developing the disease remained low.
For expert tips to help feel your best, get AARP’s monthly Health newsletter.
Like other lymphomas, ALCL is a cancer of the immune system, not breast tissue. In patients with breast implants, the disease can develop within the capsule of fibrous scar tissue that forms around implants after surgery. Experts say the disease is slow-growing and highly treatable when detected early.
The main symptoms of breast implant-associated ALCL include persistent pain, swelling or lumps in the vicinity of the implant, and the problems can appear years after the procedure. The FDA advises anyone with breast implants who is experiencing troubling symptoms to contact their health care provider. It's also important for patients to follow their physician's recommendations for regular screenings, including mammograms and magnetic resonance imaging (MRI) tests as needed.
According to the American Society of Plastic Surgeons, nearly 7,500 women age 55 and older underwent breast augmentations in 2017. The FDA recommends that those considering breast implants discuss the risks with their health care provider and review the safety data for their choice of silicone or saline implant, which the agency makes publicly available. Patients and providers are also encouraged to report any serious side effects through the FDA's MedWatch system.
"We hope,” the agency said in its report, “that this information prompts providers and patients to have important, informed conversations about breast implants and the risk” of ALCL.