Staying Fit

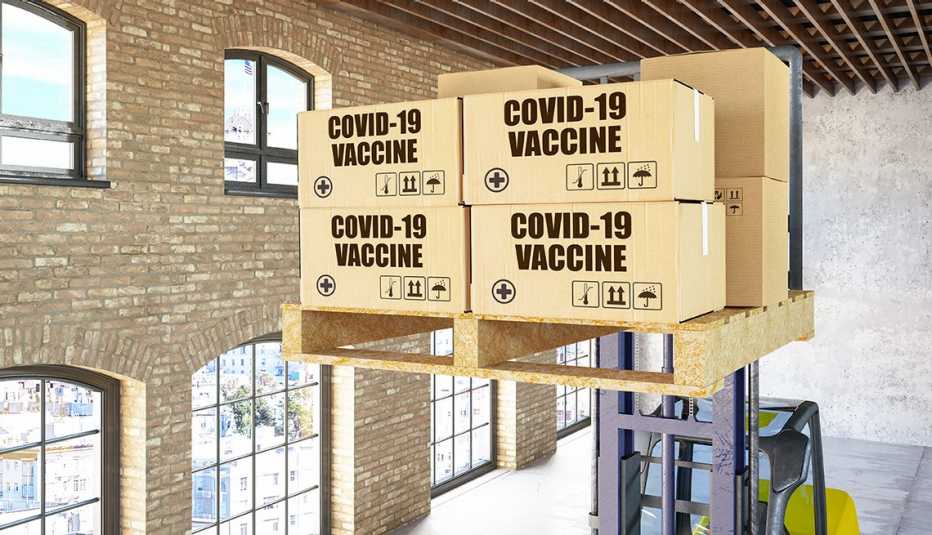
The federal government has delivered more than 29 million doses of COVID-19 vaccine to states, territories and tribal governments as of Jan. 13, but only about 10.3 million, or 35 percent, have been administered, according to tracking by the Centers for Disease Control and Prevention (CDC). While that rate has been ticking up in recent days, it still means millions of shots are sitting in storage. In a handful of states, the vaccine administration rate is 25 percent or less.
A vaccination campaign unprecedented in its complexity and scope has been slowed by several obstacles, some predictable, some not. Here are the key issues that have dogged the rollout during its first month — and some of the steps being taken to address them.


AARP Membership— $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
1. This has never been done before.
Operation Warp Speed, the partnership between the federal government and major drug companies to rapidly develop and distribute COVID-19 vaccines, reached its first goal with unrivaled success. Past vaccines like those for polio and flu took years, even decades, to develop. The Pfizer and Moderna COVID shots were created, tested and authorized for use in just nine months.
But that speed had a “downside,” said Eric Toner, M.D., a senior scholar at Johns Hopkins University’s Center for Health Security. “It leaves less time to have a carefully planned rollout of the vaccine, for both the feds to figure out how they’re going to get the vaccine to the states and for the states to figure out how they’re going to distribute it and educate the public.”
In October 2019, the center staged a simulation called Event 201 aimed at gauging American and global readiness for a worldwide pandemic. The timing was coincidental — the center had held several such exercises since 2001 involving a range of potential public health crises — but the scenario was eerily prescient, positing a new strain of coronavirus that leaps from animals to humans and spreads rapidly around the globe, stalling economies, halting travel and setting social media afire with disinformation.
But as participants gamed out the medical, financial and logistical challenges such a pandemic could (and, as it turned out, did) present, how to quickly and effectively inoculate people against this new coronavirus didn’t come up. “We never anticipated that we’d be able to have effective vaccines in less than a year,“ Toner said. “That’s never happened before.”
Add to that the particular complexities of the Pfizer and Moderna vaccines, the two authorized for use to date. Both require extremely cold storage (especially the Pfizer shot) and two doses to achieve full efficacy. “It would have been surprising if it did go perfectly,” Toner said.
2. Vaccine supply is limited and uncertain.
Dose delivery fell short of expectations almost from the start. On the campaign trail in 2020, President Trump touted a goal of 100 million vaccine doses by year-end. Even after the administration revised that target down to 20 million, many states saw their weekly allocations from the federal government reduced shortly after the initial vaccine shipments in mid-December.
“The challenge we face is that week by week, we don’t know how many doses we will receive, making it difficult to plan for more than a week in advance,” Colorado Gov. Jared Polis said this week.
That challenge has trickled down to the health care providers that are putting shots in arms. “The unpredictable nature of vaccine shipments has challenged hospital vaccination efforts, including for staff,” said Maryellen Guinan, principal policy analyst at America’s Essential Hospitals, an association of public and nonprofit hospitals serving a safety-net role. “The inability to know the exact number of doses to be delivered and the timing of deliveries directly impacts vaccine scheduling.”
The two-shot regimen has also affected supplies, with the U.S. Department of Health and Human Services (HHS) initially holding millions of doses in reserve as second shots for people who’ve gotten the first. The agency recently started releasing those doses to further enhance available stocks, in conjunction with its call for states to open up vaccination to people age 65 and over and other vulnerable populations. HHS Secretary Alex Azar said on Jan. 12 that Moderna and Pfizer are producing enough vaccine that “we can now ship all of the doses that had been held in physical reserve, with second doses being supplied by doses coming off of manufacturing lines.”
Some states said that even with increased availability they will struggle to vaccinate an exponentially larger pool of eligible people. “There are simply vastly more Georgians that want the vaccine than can get it today,” Georgia Gov. Brian Kemp said at a news conference this week. “I would prefer that we have ample supply and that we could vaccinate everyone immediately. Unfortunately, that is simply not possible.”
































































More on health
Feds Shift COVID-19 Vaccine Focus to People 65 and Over or With Underlying Conditions
States with higher share of older residents, faster vaccination pace will get more dosesOlder Americans Growing More Comfortable With Coronavirus Vaccine
Nearly half want the shots as soon as possible, poll showsWhat It's Like to Get the COVID-19 Vaccine: Older Americans Open Up
They're among the health care workers who were first to get shots