AARP Hearing Center

This article was created with the assistance of generative AI and reviewed by editors before publication.
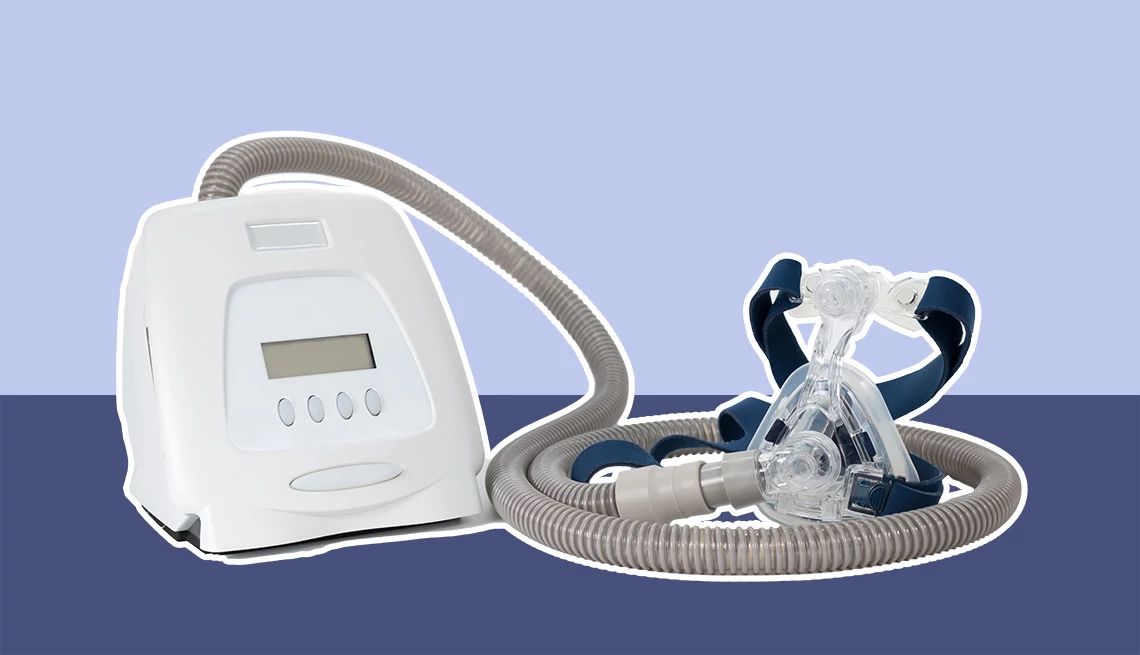
Philips Respironics has issued an urgent recall for certain BiPAP ventilator devices due to a risk of interrupted or compromised breathing support, which could lead to serious health complications—including hypoventilation, low oxygen, high carbon dioxide levels, respiratory failure or death in vulnerable patients.
The FDA has classified the recall as a Class I, its most serious type. It affects several BiPAP models commonly used for conditions like sleep apnea and other respiratory issues.
So far, 13 injuries and eight deaths have been linked to the problem.
Which BiPAP machines are affected?
The recall affects the following BiPAP ventilator models:
- BiPAP A30, Model Number: 00606959039308
- BiPAP A40, Model Number: 00606959039476
- BiPAP V30 Auto, Model Number: 00606959049635
These devices provide noninvasive or invasive support for adults and children with obstructive sleep apnea, respiratory insufficiency or respiratory failure. They are intended for use in various settings, including hospitals, sleep labs and home care.
The affected devices may:
- Intermittently reboot for 5 to 10 seconds, pausing therapy and displaying a blank screen with a single audible alert, then restart using either the patient’s settings or factory defaults.
- Enter a “ventilator inoperative” state—stopping therapy and activating audible and visual alarms—after three reboots within 24 hours, or in some cases, without any prior reboot.
These malfunctions could result in serious respiratory complications or even death in susceptible patients.
What should consumers do?
To address this issue, Philips Respironics is updating the instructions for using affected devices. Users and caregivers should follow these steps:
- If a “ventilator inoperative” alarm occurs, immediately remove the patient from the device and connect them to an alternate source of ventilation, if available.
- If therapy interruptions cannot be tolerated, provide alternate ventilation and contact your equipment supplier for an immediate replacement or alternative device.
- Optionally, you can try a “hard reboot” (a forced device restart), which may temporarily restore the machine’s function.
Philips Respironics has also sent a recall notice to patients, caregivers and clinicians with a response form to confirm they’ve taken action.
Customers in the U.S. with questions about this recall can contact Philips Respironics at 800-345-6443 or via email at respironics.clinical@philips.com.

































































More From AARP
Product Recalls and Consumer Safety
The latest alerts impacting your health and homeWhy Are You Short of Breath? 14 Explanations
It’s not a normal part of aging, and it could signal one of many health issues
Insights on Sleep Studies and Their Benefits
Overnight evaluations reveal critical sleep issues