AARP Hearing Center

The Centers for Disease Control and Prevention (CDC) is warning consumers about counterfeit botulinum toxin, commonly known as Botox, after patients in nine states reported harmful reactions from their injections.
Affected individuals showed symptoms similar to botulism near their injection sites. However, their symptoms were consistent with the toxin spreading to other parts of the body, a statement from the Food and Drug Administration (FDA) added.
Botulism is caused by a bacterium called Clostridium botulinum, which is found in nature and is an ingredient in Botox and similar products used for cosmetic purposes, according to the Illinois Department of Public Health.
All 19 individuals who reported negative reactions were administered Botox by unlicensed or untrained individuals in non-health care settings, such as homes or spas, the CDC said. The incidents occurred in Colorado, Florida, Illinois, Kentucky, Nebraska, New Jersey, New York, Tennessee and Washington.
The FDA said the counterfeit Botox had also been administered in health care settings by licensed practitioners.
“The products appear to have been purchased from unlicensed sources,” said the FDA statement. “Medications purchased from unlicensed sources may be misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective and/or unsafe.”
Of the 19 people who reported illness, four were hospitalized. Meanwhile, the FDA is working with Botox’s manufacturer, AbbVie, to identify, investigate and remove suspected products from the U.S.
Advice for consumers
Anyone considering a Botox injection for medical or cosmetic reasons should ask their provider — whether in a health care or spa setting — if they are licensed and trained to give the injection. In some states, there may be a license look-up tool, said the CDC.
Ask if the drug used for the procedure is approved by the FDA and if it was purchased through a legitimate source.
If you have any doubt, don’t get the injection, the CDC urged.

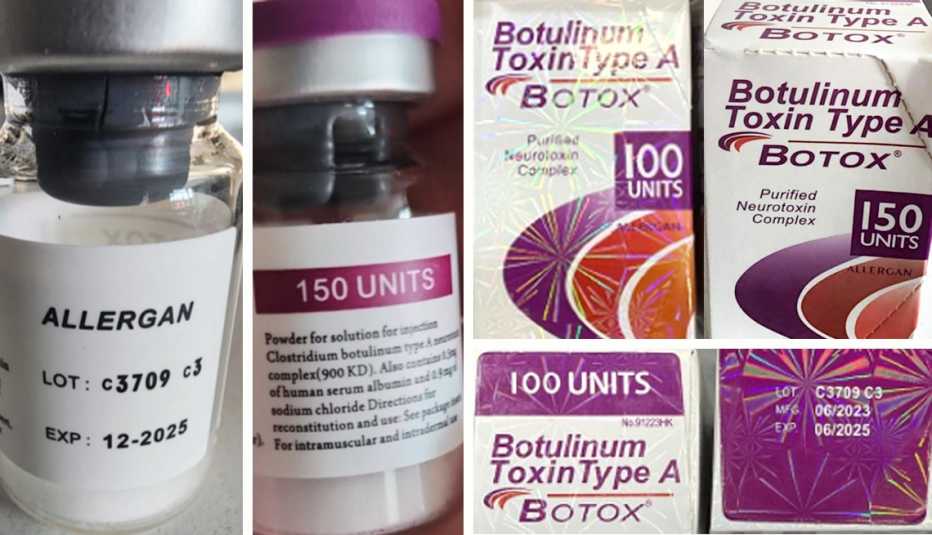
The counterfeit Botox can be distinguished from the legitimate product by certain markers on the outer carton: the lot number C3709C3, language that is not English, and if the active ingredient is listed as “Botulinum Toxin Type A” instead of the FDA-approved “OnabotulinumtoxinA.” Additionally, the carton may incorrectly state a 150-unit dose, a dosage not manufactured by the legitimate producer.
Symptoms of botulism
Anyone with symptoms that resemble botulism should go to their health care provider or the emergency room immediately, said the CDC. Symptoms include:
- Blurry or double vision
- Drooping eyelids
- Difficulty swallowing
- Difficulty breathing
- Muscle weakness
These symptoms all result from muscle paralysis caused by the toxin. If untreated, the condition may cause full paralysis of some muscles, including those used for breathing and walking.





































































More on Health
Vitamins You Can Overdo
Taking too many of these can be dangerous
Do Injections for Knee Pain Work?
The shots are common, but results can vary
Are You Using Your Neti Pot Correctly?
Never practice nasal rinsing with tap water, experts say
Try These Tips for Living a Healthier Life
Small changes can add up to big mental and physical results
Recommended for You