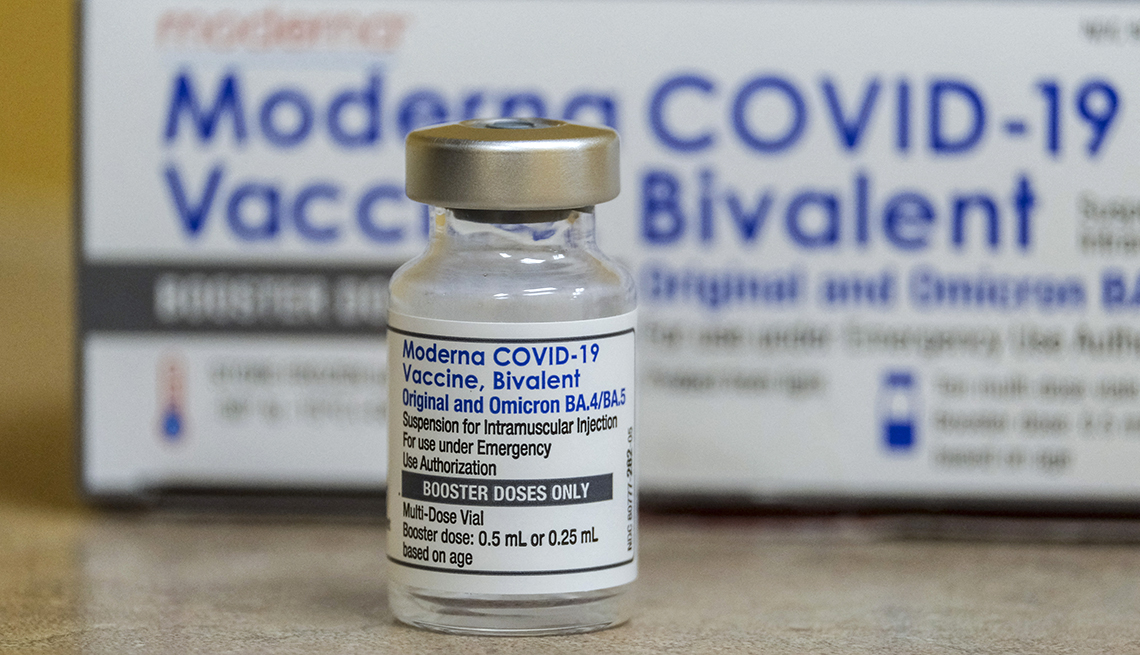AARP Hearing Center
Adults 65 and older who received what’s known as a bivalent COVID-19 booster at least four months ago are eligible for a second dose, following authorization from the U.S. Food and Drug Administration (FDA) and sign off from the Centers for Disease Control and Prevention (CDC).
People with compromised immune systems have also been cleared for a second jab of the bivalent shots.
The bivalent boosters from vaccine makers Moderna and Pfizer-BioNTech first became available in September 2022. They target both the original strain of the coronavirus and subvariants BA.4 and BA.5 of the omicron strain, which has been circulating globally for more than a year.
Even though BA.4 and BA.5 have been eclipsed by other omicron subvariants, studies show the bivalent shots still provide protection against currently circulating strains. Federal data from January 2023 shows that individuals who received this updated shot are almost eight times less likely to die from COVID-19 than unvaccinated individuals, and they’re 1.7 times less likely to die from the illness than vaccinated individuals who did not get the bivalent booster. Hospitalization rates are also lower for people who received the bivalent booster compared with those who didn’t.
Research shows, however, that the strength of the vaccine’s protection against infection and illness wanes some over time. In an April 18 call with reporters, Peter Marks, M.D., director of the FDA’s Center for Biologics Evaluation and Research, said that “barring the development of a radically new [coronavirus] variant,” the data suggests that a six-month interval between shots is reasonable for older individuals, who are at higher risk for severe illness from a coronavirus infection. More than 93 percent of people who have died from COVID-19 have been 50 or older.
People who are immunocompromised can get a second bivalent booster two months after their first bivalent vaccine, health officials said in the most recent updates, and any additional doses can be administered at the discretion of their health care provider.