AARP Hearing Center
Drugmaker Aurobindo Pharma recalled two lots of the blood pressure medication Quinapril-Hydrochlorothiazide after levels of the cancer-causing impurity nitrosamine was detected above the acceptable daily intake level set by the Food and Drug Administration (FDA).
Elevated levels of a nitrosamine impurity, Nnitroso-quinapril, were found in 20 mg and 12.5 mg tablets of the drug. The recalled batches were shipped nationwide in May 2021 and have a January 2023 expiration date. The recalled lot numbers are QE2021005-A and QE2021010-A.
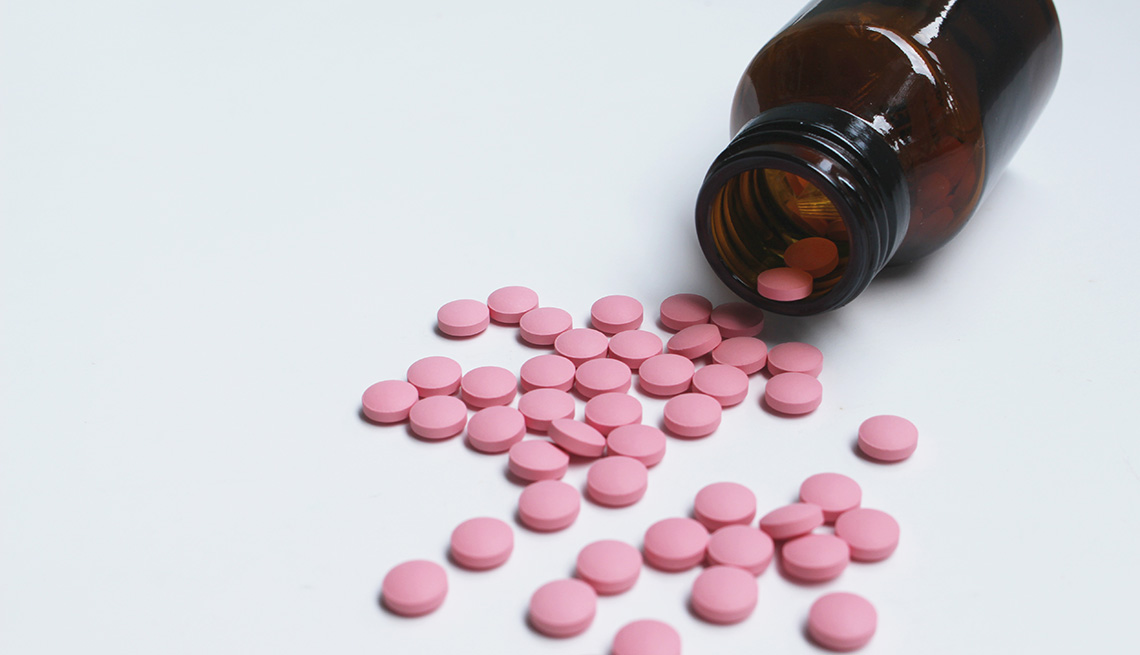
The pills are round, pink film-coated tablets, with a D on one side and a 19 on the other. To date, there have been no reports of anyone having negative reactions from the recalled drugs.
Advice to consumers
Patients taking the recalled tablets should contact their health care provider to discuss whether to continue taking the drug or consider an alternative treatment before returning their medication. Aurobindo Pharma will notify distributors and customers by phone and in writing to stop distribution of the specific lots and provide instructions for returning the recalled products.
Consumers with medical questions regarding this recall or wishing to report an adverse event can contact the manufacturer at 866-850-2876, or email pvg@aurobindousa.com. Questions about returning the drug can be made to Qualanex at 888-504-2014.
Negative reactions or quality problems may also be reported to the FDA’s MedWatch Adverse Event Reporting program online, by phone at 800-332-1088 or by fax using this form to 800-FDA-0178.
What are nitrosamines?
Long-term ingestion of Nnitroso-quinapril may be associated with an increased cancer risk in humans.
Nitrosamines are present in water and foods such as cured and grilled meats, dairy products and vegetables. Although everyone is exposed to some level of nitrosamines, the FDA set an internationally recognized acceptable daily intake limit for the impurity. The FDA recommends that drugs containing levels above the acceptable daily intake limit be recalled as appropriate.
A person taking a drug that contains nitrosamines at or below the acceptable daily limit every day for 70 years is not expected to have an increased cancer risk, according to the FDA.