Have AFib? Put Science on Your Side
Help reduce your risk of stroke. Here’s how.
Do you or a loved one have atrial fibrillation? This condition, also known as AFib, puts you at a 5 times higher risk of suffering a stroke than someone with a regular heartbeat.1 Until recently, the only way to treat the increase in stroke risk was with oral anticoagulants, or blood thinning medication.
In 2015, the FDA approved the WATCHMAN™ Left Atrial Appendage Closure Implant, a one-time procedure that could reduce non-valvular AFib related stroke risk for a lifetime without the need for long-term blood thinners. The implant continues to advance with the latest generation, called the WATCHMAN FLX™ Implant, approved by the FDA in 2020.
If the idea of an implant for reducing stroke risk is new to you, you can feel comfortable knowing that more than 150,000 implant procedures have been performed worldwide. With almost 20 years of clinical trial and real-world experience (including 10 clinical trials), this treatment has a proven safety record.
How This Stroke Risk Reduction Treatment is Different
While blood thinners can be an effective treatment for AFib stroke risk, certain risks and downsides associated with extended use of blood thinners may make patients think twice before committing. In fact, more than a third (38%) of those taking oral anticoagulants feel trapped between their fear of having a stroke and their fear of the risks associated with these blood thinners.2
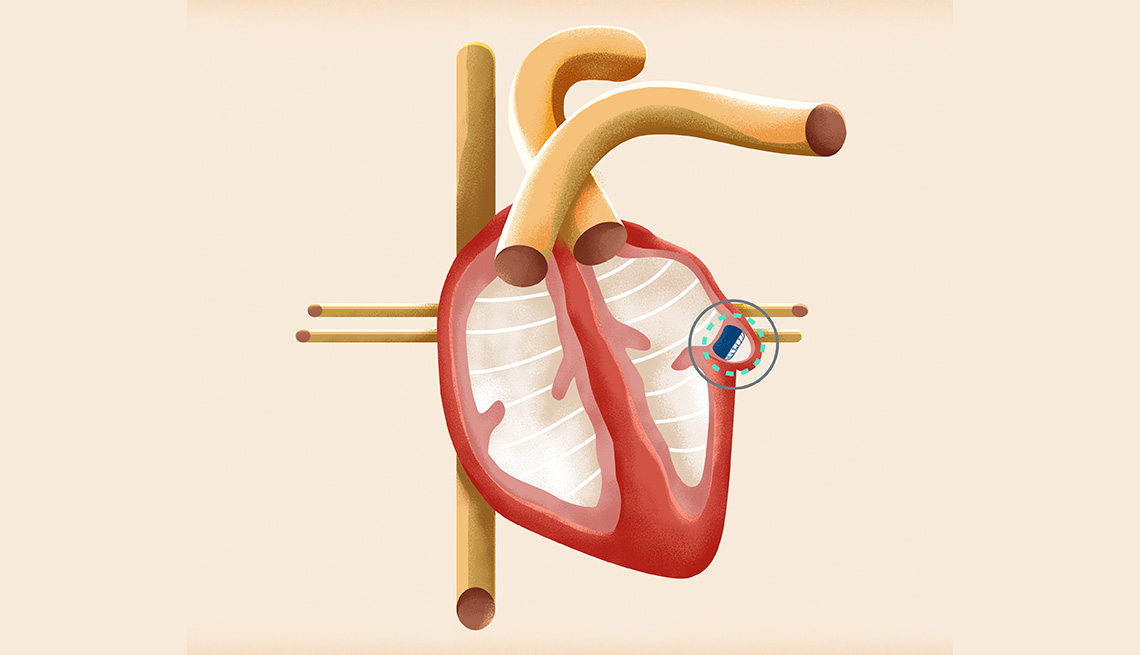
The WATCHMAN Implant offers an effective alternative to blood thinners by closing off the part of your heart called the left atrial appendage or LAA, where stroke-causing clots are most likely to form. In people with AFib not caused by a heart valve problem, more than 90% of stroke-causing clots that come from the heart are formed in the LAA.3
The implant fits right into your LAA. It’s designed to permanently close it off and keep those blood clots from escaping. The size of a quarter, it’s made from very light and compact materials commonly used in many other medical implants.
How the Implant Procedure Works
The implant is placed into your heart in a minimally invasive, one-time procedure typically done under general anesthesia. The procedure takes about an hour, and patients usually stay in the hospital overnight and leave the next day. Over time, your left atrial appendage will completely close off to prevent blood clots from traveling.
Eventually, you’ll likely be able to stop taking blood thinners; in fact, 96% of people were able to stop taking blood thinners just 45 days after the procedure.4 It’s the most implanted device approved by the FDA to reduce the risk of stroke safely and effectively in people with atrial fibrillation not caused by a heart valve problem.
Who the Implant is For
The WATCHMAN Implant may be right for people who have atrial fibrillation not caused by a heart valve problem (also known as non-valvular AFib). The implant is also ideal for those who need an alternative to blood thinners for their stroke risk reduction. That might include people who have had major bleeding while taking blood thinners or those who have a lifestyle, occupation, or condition that puts them at risk for major bleeding. It might also cover anyone who has difficulties taking their blood thinner as prescribed, due to issues such as staying in INR range, following dietary restrictions, missing doses, or inability to afford the prescription.
With the WATCHMAN Implant, you’re free to live life on your terms, whether that means going for a family bike ride or planning that long-awaited dream trip to the other side of the world. You’ll no longer feel stifled by the risks associated with blood thinners. You’ll be ready to start enjoying the things you love doing again.
Click here to take this short survey to see if the WATCHMAN™ Implant is right for you or a loved one.
Important Safety Information: The WATCHMAN and WATCHMAN FLX Devices are permanent implants designed to close the left atrial appendage in the heart in an effort to reduce the risk of stroke.
With all medical procedures there are risks associated with the implant procedure and the use of the device. The risks include but are not limited to accidental heart puncture, air embolism, allergic reaction, anemia, anesthesia risks, arrhythmias, AV (Arteriovenous) fistula, bleeding or throat pain from the TEE (Trans Esophageal Echo) probe, blood clot or air bubbles in the lungs or other organs, bruising at the catheter insertion site, clot formation on the device, cranial bleed, excessive bleeding, gastrointestinal bleeding, groin puncture bleed, hypotension, infection/pneumonia, pneumothorax, pulmonary edema, pulmonary vein obstruction, renal failure, stroke, thrombosis and transient ischemic attack. In rare cases death can occur.
Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the device.
1 Holmes D. Atrial Fibrillation and Stroke Management: Present and Future. Semin Neurol 2010, 30:528-536.
2 WatchUsNow.com. The Harris Poll online survey. Boston Scientific. SH-574213-AA. https://www.watchusnow.com/?page=d75be9d4-ba36-456c-a72d-dc3df07892da. Accessed March 28, 2019.
3 Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755-759.
4 Kar, S., et al, Primary Outcome Evaluation of the Next Generation LAAC Device: Results from the PINNACLE FLX Trial, Circulation, 2021.