Staying Fit

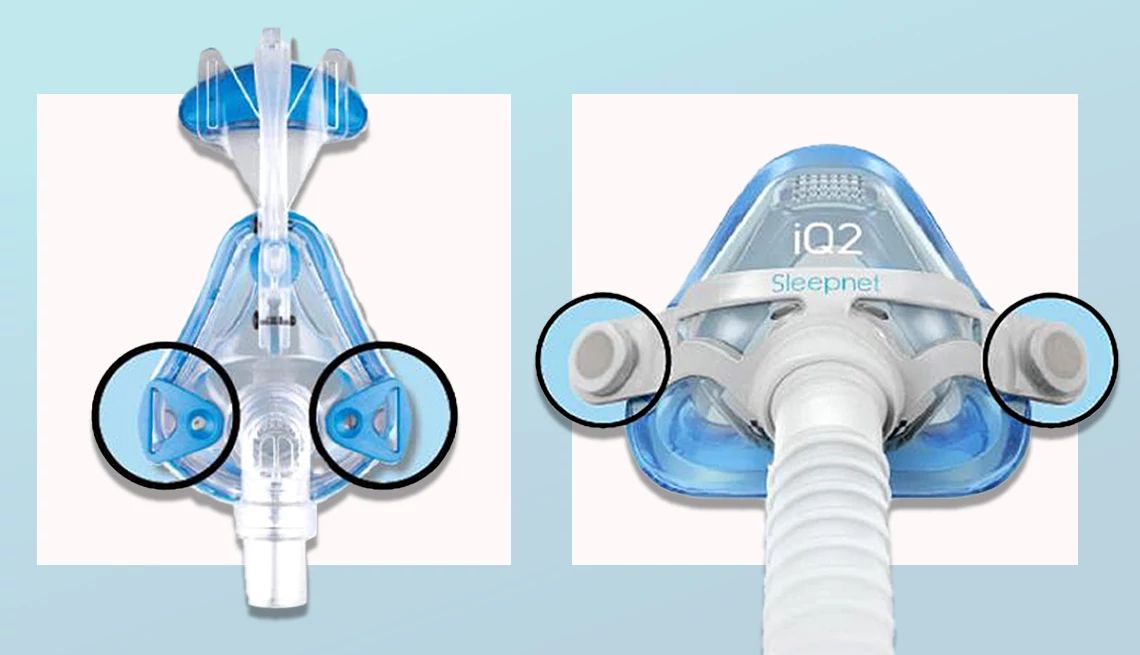
Sleepnet Corporation recalled all its CPAP and BIPAP sleep apnea masks that have magnets because they may interfere with certain medical devices, according to a March 18 announcement from the Food and Drug Administration (FDA).
When magnets are near certain medical or metallic implants, they may interfere with the performance or position of the implant and result in serious injury or death, the notice said.


AARP Membership— $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
The company stated that while it has been selling masks with magnets globally since 2006, there have been no reported adverse events linked to Sleepnet masks with magnets.

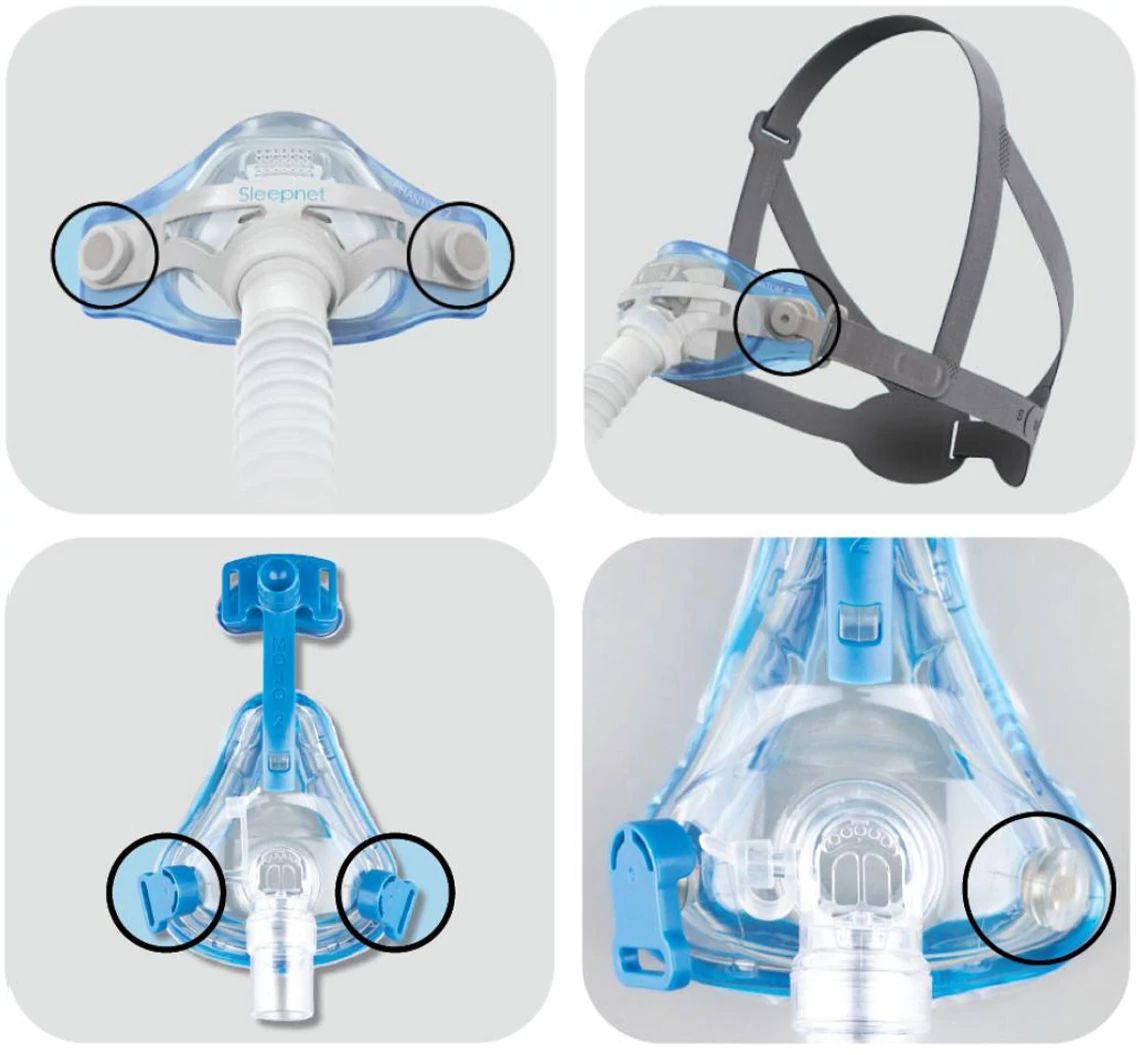
The recalled sleep apnea masks
All lot and unique device identification numbers of the following products are recalled: Mojo Full Face Vented Mask, Mojo Full Face Non‐Vented Mask, Mojo 2 Full Face Vented Mask, Mojo 2 Full Face Non‐Vented Mask, Mojo 2 Full Face AAV Non‐Vented Mask, iQ 2 Nasal Mask and Phantom 2 Nasal Mask.
Advice to consumers
The manufacturers caution not to use the masks if the patient or anyone in close contact with the mask has any of the following active medical or metallic implants: pacemakers, implantable cardioverter defibrillators (ICD), neurostimulators, aneurysm clips, metallic stents, ocular implants, insulin/infusion pumps, cerebral spinal fluid (CSF) shunts, embolic coils, metallic splinter, implants to restore hearing or balance with implanted magnets (such as cochlear implants), flow disruption devices, contact lenses with metal, dental implants, metallic cranial plates, screws, burr hole covers, bone substitute device, magnetic metallic implants/electrodes/valves placed in upper limbs, torso or higher.
Anyone with implants or other medical devices sensitive to magnets should stay at least 6 inches away from the masks, the company added in an updated warning.
Anyone who thinks the magnets in Sleepnet masks and headgear clips might affect them should find a replacement that does not include magnets, or speak with their physician or the manufacturer of their implant to discuss any questions.
No additional information about replacement masks or refunds was provided.
Consumers with questions may contact Sleepnet at hkoppusetty@sleepnetcorp.com or call 1‐800‐742‐3646.



































































More on Sleep
Why Missing Sleep Is Worse for Women Than Men
Not only do females get less sleep than males, their health suffers more from it
How to Create a Sleep Sanctuary and Other Tips for a Good Night's Sleep
13 habits to give your body the rest it needs
6 Simple Strategies for Sleeping Through the Night
Tips for getting the deep sleep you need. Plus, how to get back to sleep if you wake in the wee hours
Recommended for You