AARP Hearing Center
Health
The latest science-based health news on fitness, nutrition, medications, medical breakthroughs and more. Plus, tips on how to live your healthiest life at 50 and beyond.
AARP Tools to Keep You Healthy
Events Recommended For You


AARP Membership
Join AARP for $15 for your first year when you enroll in automatic renewal. Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
Trending in Health


Conditions and Treatments
Is the Heat Giving You a Headache?
High temps can trigger migraines. How to prevent them


Conditions and Treatments
Lithium Reverses Memory Loss in Mice
Test results offer promise in treating Alzheimer's


Conditions and Treatments
Advances in Pancreatic Cancer Treatment
Know the warnings signs to diagnose it early

Conditions and Treatments
What is Legionnaires' Disease?
2 have died. Nearly 60 are sick in NYC outbreak

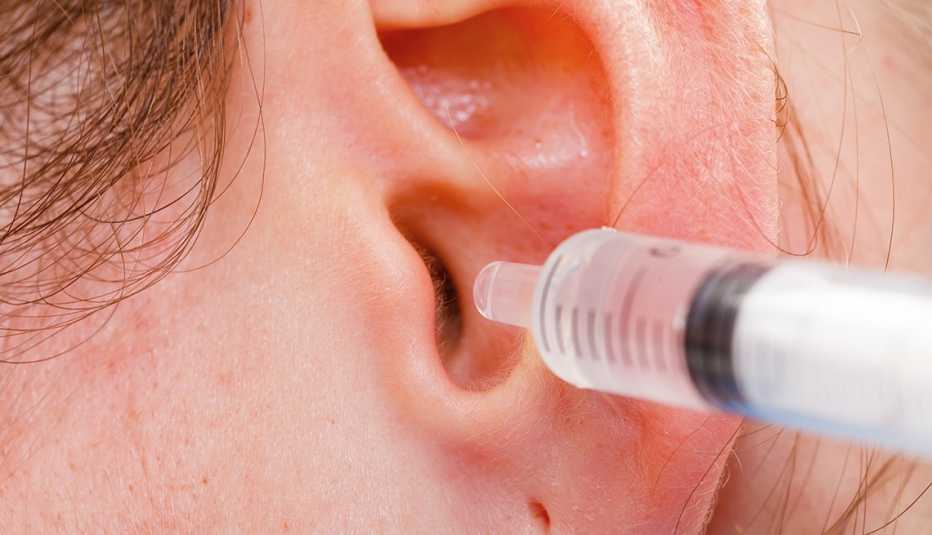
Conditions and Treatments
Tips on Safely Removing Earwax
Advice on at-home earwax removal from the experts
AARP IN YOUR STATE
Find AARP offices in your State and News, Events and Programs affecting retirement, health care and more.


Tips For Living Your Best Life
When it comes to your health and well-being, knowledge is power. That’s why AARP has the latest, science-backed information on habits that support your health as you age, exercises that benefit your body and mind, and so much more.
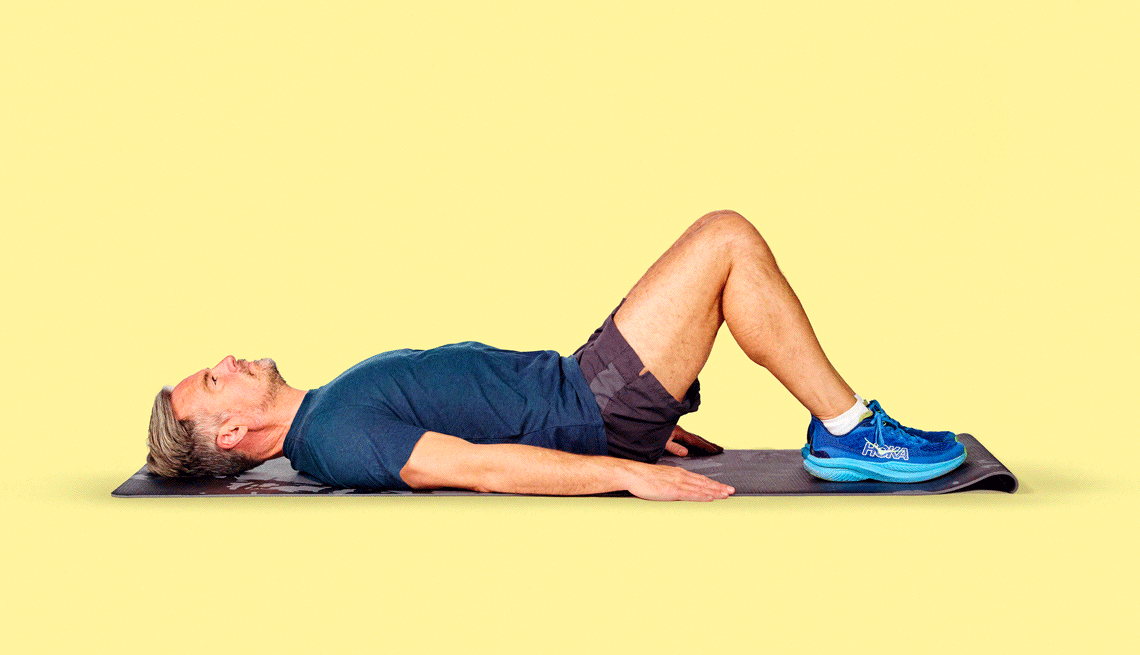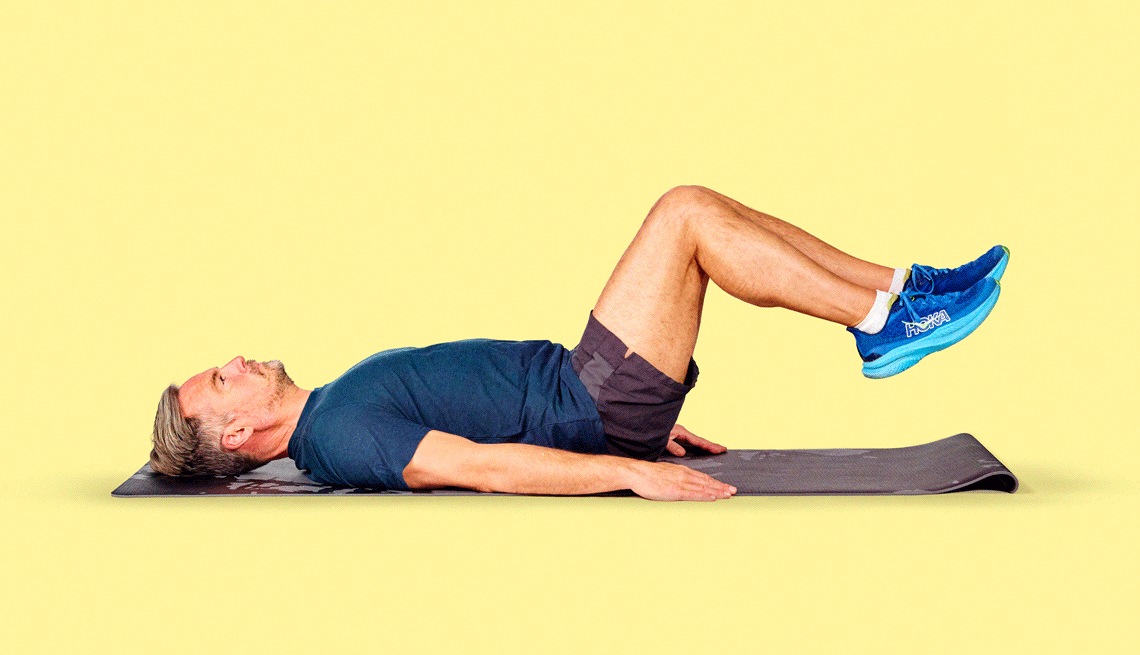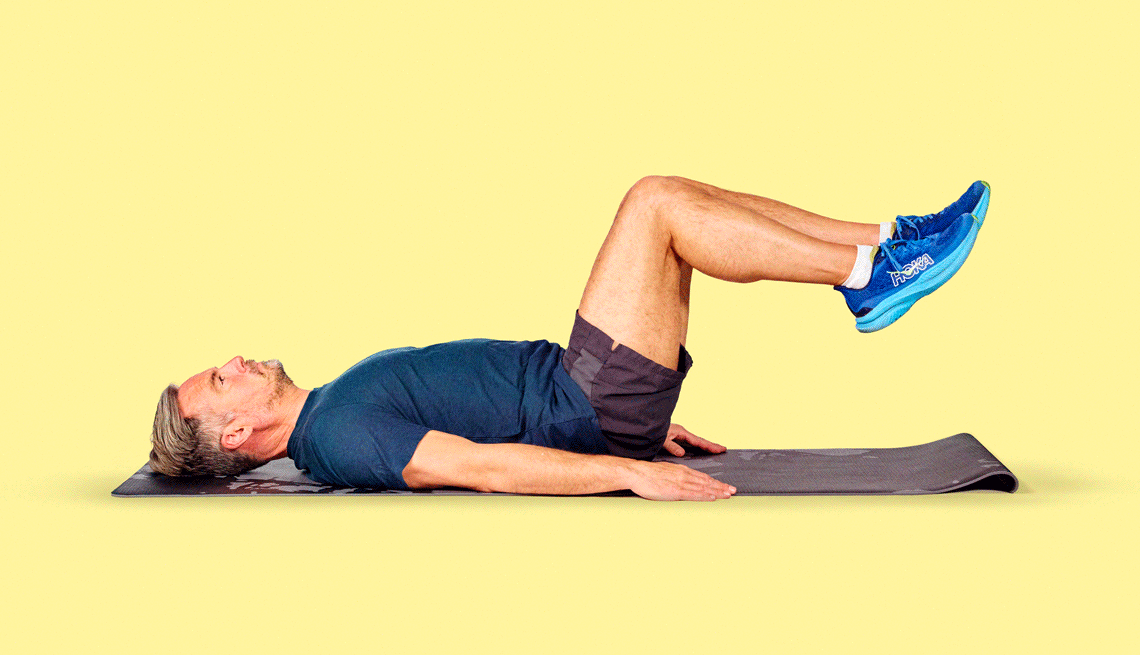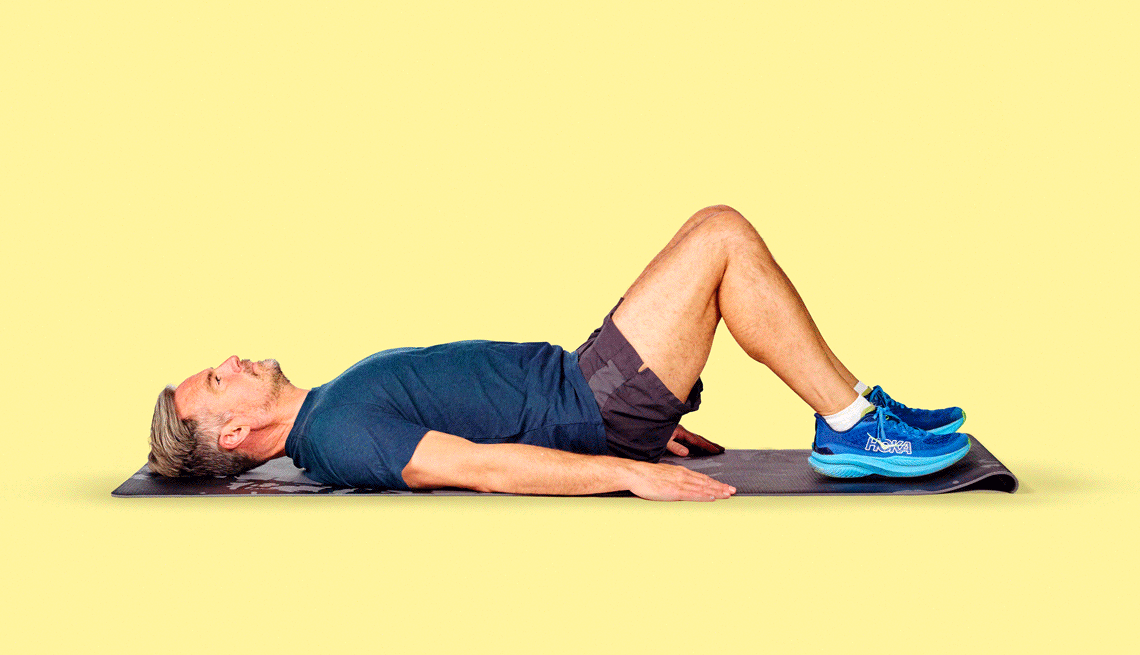
Get inspired with workout routines and videos with exercises for every fitness level.
Learn all about the joy of the sport — how to score, tips for playing, ways to win and more.
Information to help you understand brain health and build awareness through research, reports and tips.