Staying Fit
Two more brands of eye drops have been recalled due to contamination risks that could cause adverse reactions, including eye infections that could lead to blindness.
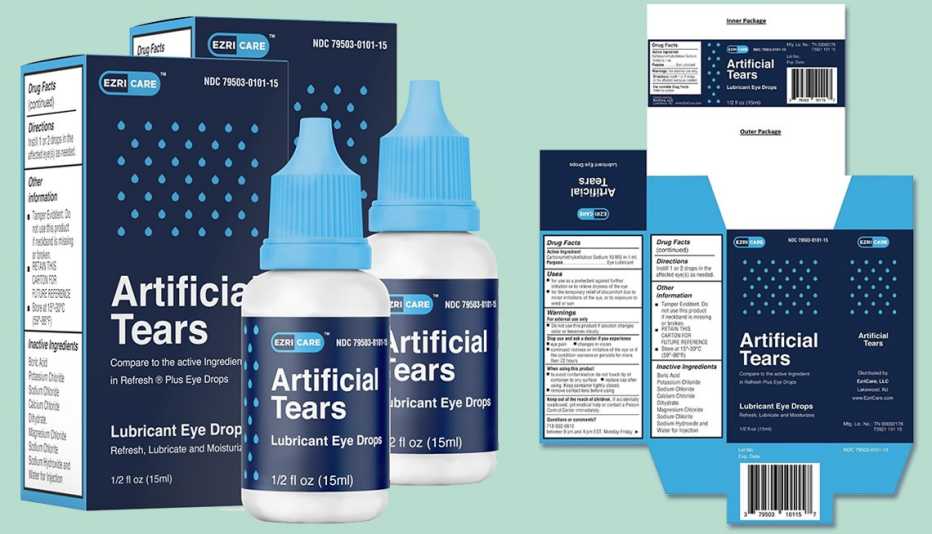
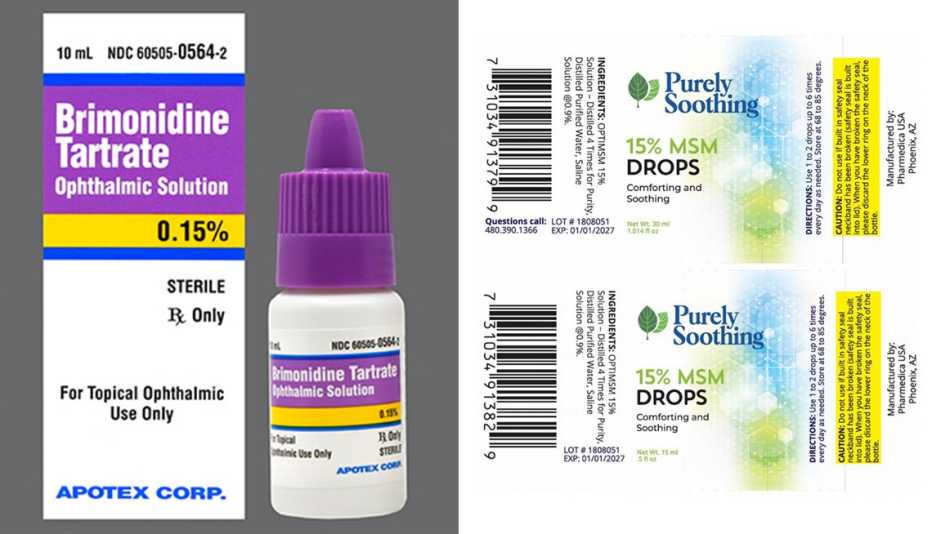
The Food and Drug Administration (FDA) issued recall notices for eye drop manufacturers Pharmedica and Apotex, citing contamination concerns. In February, EzriCare Artificial Tears were recalled after being linked to antibiotic-resistant infections. At least 55 cases were reported, which resulted in vision loss, hospitalization and one death.


AARP Membership— $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
Two lots of Pharmedica’s Purely Soothing 15% MSM Drops, used to treat eye inflammation and irritation, were recalled due to non-sterility. The products were sold worldwide at trade shows and at online retailers, including Amazon.com. Pharmedica advises consumers to immediately stop using the recalled products and return them to the place of purchase. Questions can be directed to the company by phoning 623-698-1752 from 8 a.m. to 5 p.m. MT or by email to osm@pharmedicausa.com.
The recalled batches include lot #2203PS01, sold in a 1-ounce bottle, and lot #1808051, sold in half-ounce bottles.
Apotex pulled six lots of its Brimonidine Tartrate Ophthalmic Solution, 0.15% because the bottle caps may be cracked, causing the solution to become unsterile. The product is used by patients with open-angle glaucoma or ocular hypertension. Patients who purchased the recalled lots should contact their pharmacy and their health care provider for medical advice. The drops may be returned to Inmar Rx Solutions by calling 855-275-1273 from 9 a.m. to 5 p.m. ET.
The affected lot numbers are TJ9848 and TJ9849 with an expiration date of February 2024, and TK0258, TK5341, TK0261 and TK0262 with an April 2024 expiration date.

There have been no reports of injury or adverse events related to either of the newly recalled eye drops. There are no known connections to the February recall of Ezricare Artificial Tears.
Adverse reactions or quality problems from the use of these products may be reported to the FDA’s MedWatch adverse event reporting program, online, by regular mail or by calling 800-332-1088.