Staying Fit
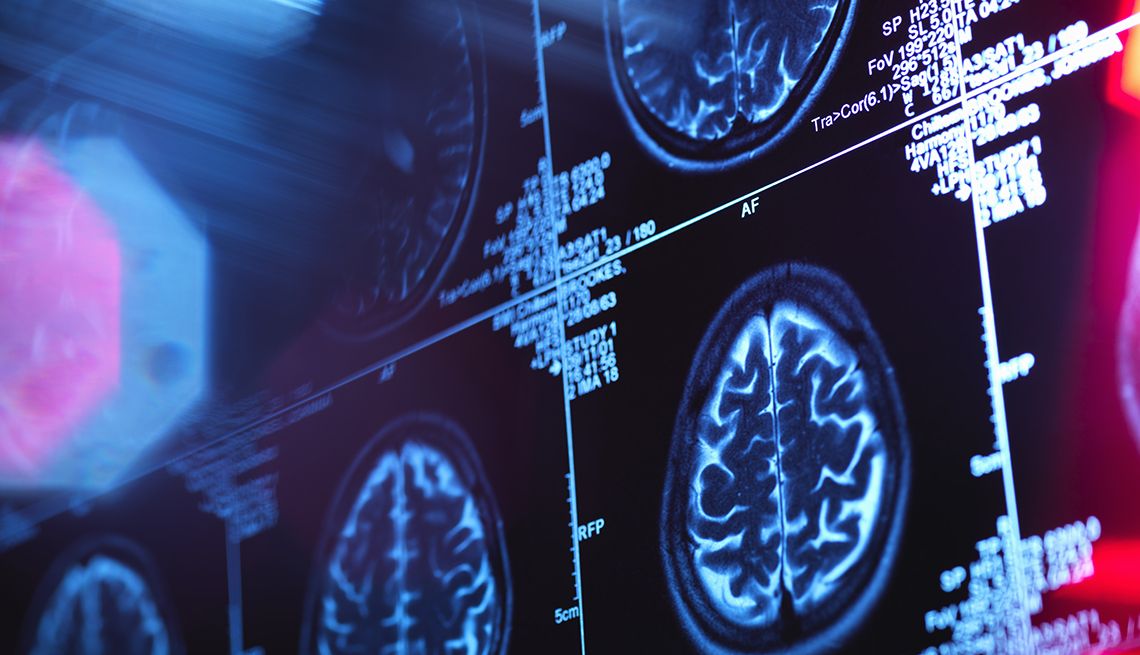
Treatment for Alzheimer's disease, the devastating condition that affects more than 5 million Americans, has remained notoriously elusive for decades. Now, a small study released by drug company Eli Lilly offers at least a flicker of hope: It shows that the experimental drug donanemab may significantly slow patients’ cognitive decline.
The two-year study — which followed 272 people whose brain scans showed Alzheimer's — found that patients who took the drug had a 32 percent slower rate of decline than those who received a placebo. “It's very encouraging because this is the first time a drug of its kind has had positive results in early-stage trials,” says Lon Schneider, M.D., Della Martin chair in psychiatry and neuroscience at the Keck School of Medicine of the University of Southern California. The drug, known as a monoclonal antibody, works by binding to the hard plaque in the brain made from amyloid (a protein associated with Alzheimer's).


AARP Membership— $12 for your first year when you sign up for Automatic Renewal
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
Though these initial findings are promising, Schneider says more data is needed. “It may have been everyone just had a small cognitive decline, in which case the results aren't as significant,” he says. (The drugmaker has said it will release this information shortly in a peer-reviewed clinical journal.)
But this isn't the only news Alzheimer's researchers are excited about. “There are several new drugs either close to getting FDA approval, or in development, that promise to really change the playing field when it comes to treatment of Alzheimer's disease,” says Marwan Sabbagh, M.D., director of the Cleveland Clinic Lou Ruvo Center for Brain Health in Las Vegas. Here are some of the most promising contenders.